Learn more about Tiziana Tiziana Investor Deck Scientific Presentations and Updates
05 April 2022
25 March 2022
New York, March 25, 2022 – Tiziana Life Sciences (Nasdaq: TLSA) ("Tiziana" or the "Company"), a biotechnology company...
Read more16 March 2022
Bioworld have published an article on Tiziana Life Sciences SPMS 6-month data using intranasal foralumab.
Read moreOur mission is to bring breakthrough therapies to patients with the aim of treating SPMS, Crohn's Disease, lung diseases and optimizing health outcomes.
Learn moreOur major clinical assets are supported by extensive worldwide issued patents and pending patent applications covering composition of matter, formulation technologies, manufacturing processes and disease indications.
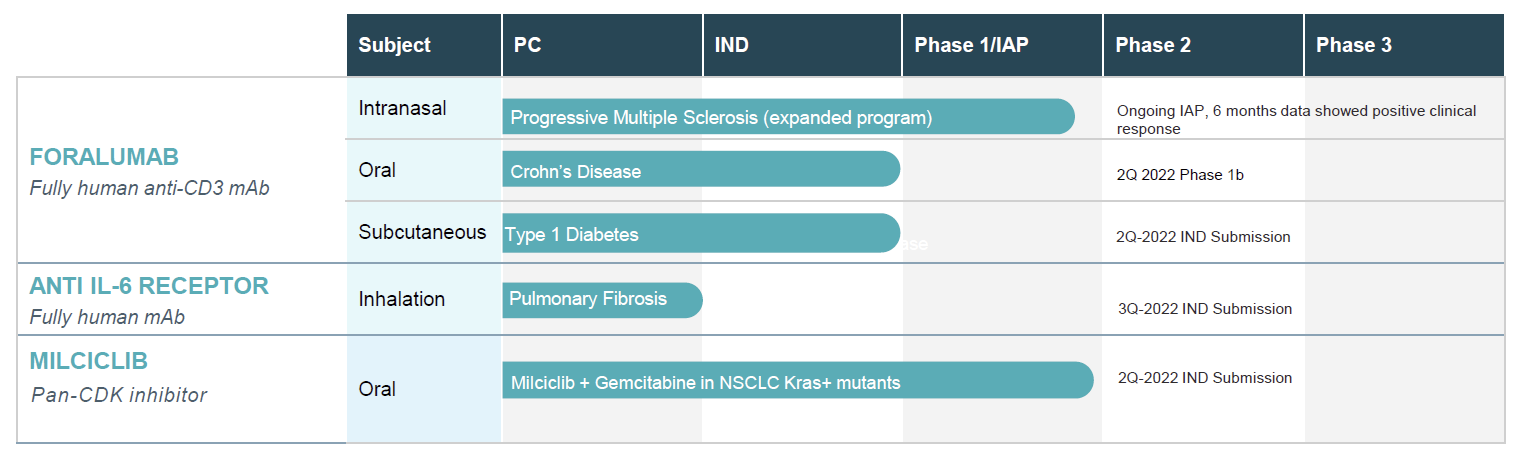
Foralumab is a fully human anti-CD3 monoclonal antibody (mAb) for treatment of Crohn’ s Disease and neurodegenerative indications.
Learn more about ForalumabWe are developing a fully human monoclonal antibody (mAb) targeting the receptor for IL-6 as a potential treatment for lung diseases.
Learn more about Anti IL-6RMilciclib is the Company’s Phase 2 clinical candidate for the treatment in cancer indications. The company is exploring the combination of milciclib and gemcitibine in NSCLC patients with pan KRAS+ mutations.
Learn more about MilciclibTiziana is currently conducting clinical development programs for Foralumab, Anti IL-6R and Milciclib
Tiziana reported postive clinical data in a Phase I clinical trial of nasally-dosed Foralumab in healthy subjects in collaboration with Dr. Howard Weiner at Brigham and Women’s Hospital in Boston. Phase 2 for both orally administered Foralumab for the treatment of Crohn's disease and nasally administered Foralumab for the treatment of Pro-MS is anticipated to be complete in Q2 2021.
See more Foralumab clinical trialsManufacturing of clinical supplies for a Phase 1 study is anticipated to be completed in 3Q/4Q 2022 and we plan to initiate a Phase 2 trial in Q4 2022/1Q 2023.
We are exploring a study to evaluate the combination of milciclib and gemcitabine in NSCLC subjects with associated pan KRAS-positive mutations.
See more Milciclib clinical trials