Crohns Disease
Crohn’s disease is a relapsing, transmural inflammatory disease of the gastrointestinal mucosa that can affect all parts of the intestinal tract as well as extra-intestinal organs. Crohn’s disease affects between 400,000 and 600,000 people in North America. Current estimates for Northern Europe have ranged from 27–48 people per 100,000.
Although the exact etiology remains unknown, the occurrence of Crohn’s disease is strongly associated with mutations of a receptor for microbial pathogens (nucleotide-binding oligomerization domain containing protein 2 - NOD2) that recognizes microbial pathogens leading to increased activation of antigen presenting cells. As a result of this altered balance of immune homeostasis, exposure to commensal bacterial antigens causes increased stimulation and proliferation of mucosal T-lymphocytes, leading to inflammatory responses.
The pharmacological management of Crohn’s disease is based on the control of the inflammatory process. The current leading biologic immunotherapies, Humira® (adalimumab) and Remicade® (infliximab) are administered parenterally to target Tumor Necrosis Factor (TNF) to induce and maintain disease remission.
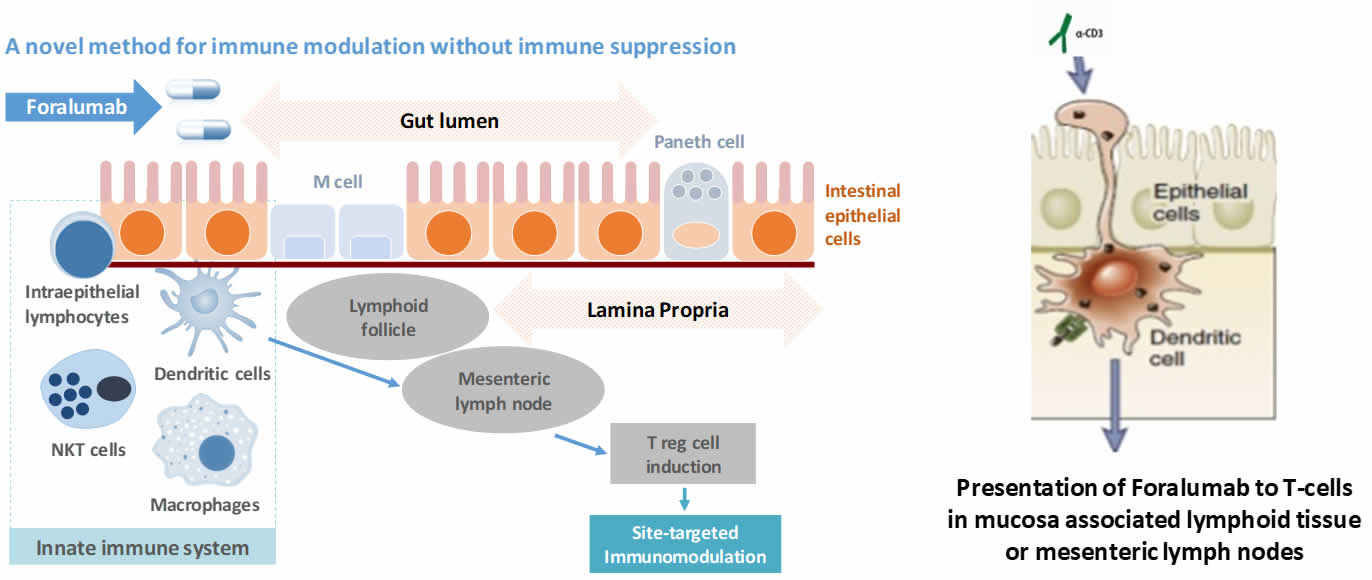
Tiziana’s intends to orally administer Foralumab to the small and large intestine via an enteric-coated capsule formulation. Foralumab binds locally to dendritic cells (antigen-presenting cells) lining the GI tract, inducing regulatory T cells supporting immunomodulation locally as well as systemically, it also has the ability to act topically to produce anti-inflammatory effects. Tiziana has completed a Phase 1 trial, conducted at the Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA. The trial was a single-site, double-blind, placebo-controlled, single ascending dose ("SAD") study in healthy subjects in which Foralumab was orally administered at 1.25, 2.5 and 5.0 mg per dose as enteric-coated capsules. Each cohort comprised of 4 subjects, of whom 3 received Foralumab treatment and 1 received a placebo capsule. All subjects completed the trial without any safety concerns at any of the doses. A Phase 1b multiple ascending dose study in mild-to-moderate Crohns patients is slated to begin in Q2 2022.
Tiziana has been granted the first-ever patent on the transformational technology for Oral Delivery of all Anti-CD3 monoclonal antibodies for treatment of human diseases.